Multiphoton Imaging
For the past two decades, biological imaging has benefited from the widespread use of femtosecond, ultrafast laser technology. Both femtosecond (10 -15 s) and picosecond (10 -12 s) pulses induce unique physical processes within biological samples that provide valuable insight inaccessible through conventional confocal imaging. The primary motivation for choosing multiphoton imaging over conventional confocal imaging is the ability to image deeper into opaque samples like brain tissue. How is this possible and what experimental conditions are required for successful multiphoton imaging experiments?
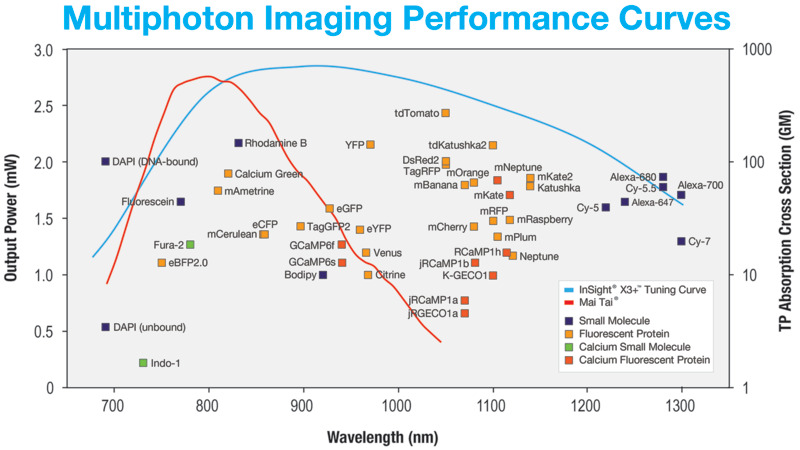
Multiphoton images are produced through simultaneous absorption of two or more photons from a wide range of fluorescent dyes. Over the past decade, several fluorescent dyes have emerged that extend the working multiphoton imaging spectral range to (680 – 1300 nm). A summary of these dyes can be found in Figure 1. The dyes are used as fluorescent markers to investigate specific biological processes and structures. Because the multiphoton absorption process is correlated to regions where photon density is highest, the resulting fluorescence is localized at the focal plane. No mechanical aperture is needed to reject out-of-plane fluorescence signal which means less scattering and less photodamage. Also, because multiphoton imaging employs near-infrared excitation wavelengths, three-dimensional images can be generated more than 1 mm into the sample.
Evolution of Multiphoton Imaging
Since the early 1990’s, Titanium Sapphire (Ti:sapphire) lasers have been the preferred light source for multiphoton imaging. The all Solid-state architecture provides robust and dependable operation across the near-IR spectral region (700 – 1000 nm). When applied to multiphoton imaging, the working absorption region translates to (~350 – 500 nm) for 2-photon and (~233 – 333 nm) for 3-photon processes - an ideal range for a wide range of biologically relevant fluorophores.
The most popular fluorescent proteins are optimally excited in the two-photon regime at wavelengths above 900 nm: eGFB (930 nm), eYFP (960 nm) and red-shifted proteins best excited at 1000-1100 nm such as mCherry, tdTomato, DSRed and E2-Crimson.
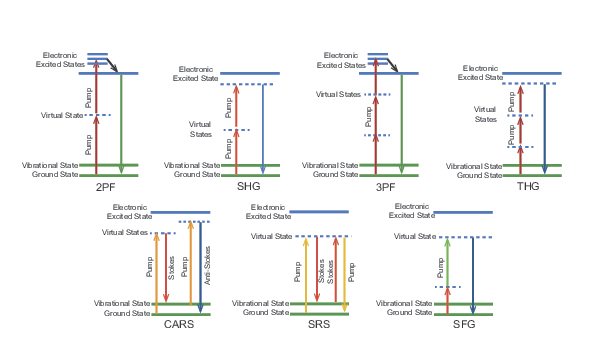
The latest generation of lasers for multiphoton imaging are based on Ytterbium (Yb). These light sources utilize nonlinear frequency conversion to achieve a working range of 680 – 1300 nm. In addition to a tunable output, these new lasers provide a high-power fixed wavelength output which has enabled new label-free techniques such as second-harmonic generation (SHG), third-harmonic generation (THG), coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering (SRS). Such label-free modalities can reveal endogenous structures naturally present in biological samples and, when used together, provides a more detailed picture when associated conventional multiphoton imaging, see Figure 1 below.
Multimodal Imaging
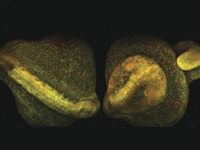
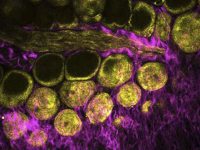
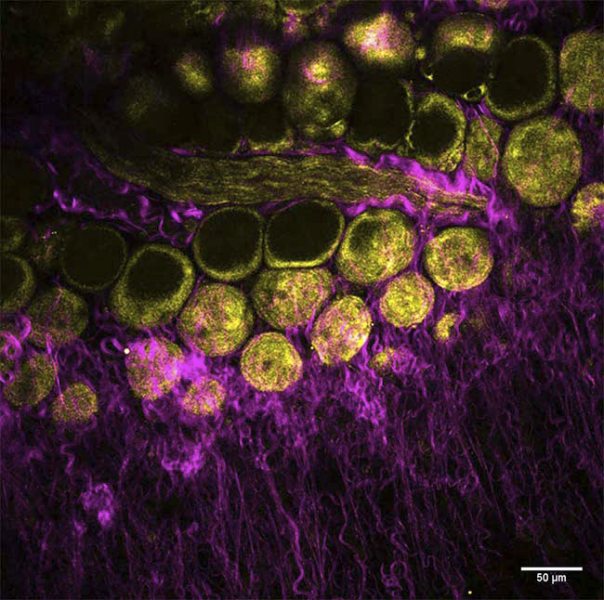
By incorporating dual-output beams to enable simultaneous excitation, new platforms are ready to meet the ever-changing needs of Multimodal imaging. Dr. Marie Irondelle at the Institute Curie/CNRS in Paris studies tumor development in the mammary gland (See figure 2). In this ex vito experiment, a mouse mammary gland was imaged using no fluorescent dyes. Irondelle simultaneously observed collagen with SHG imaging (in magenta) and adipocytes with THG imaging (in yellow).
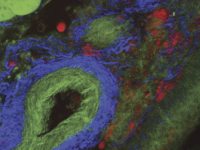
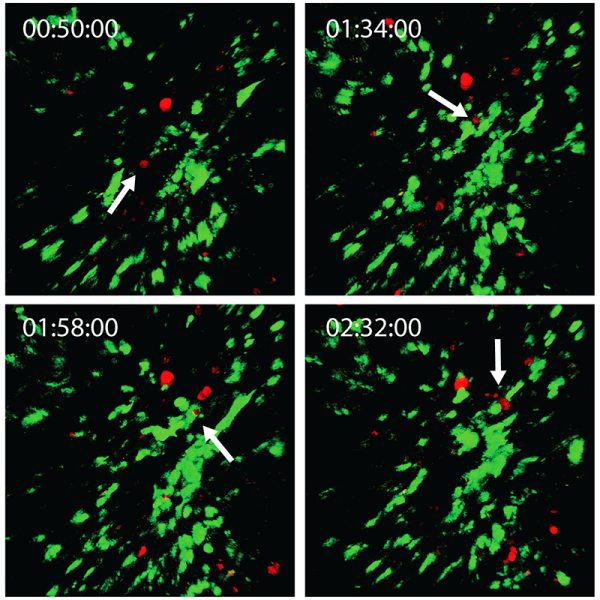
Multimodal imaging modalities can be very useful for performing in vivo experiments. Dr. Michael Kuligowski of the Centenary Institute of Cancer Medicine and Cell Biology in Sydney investigates the interaction between T cells and dendritic cells in the skin (See figure 3). Here, T cells labeled with CMTPX, were taken from a donor mouse and injected into another mouse with dendritic cells labeled with GFP. The GFP-labeled dendritic cells were image with the Mai Tai® DeepSee™ Ti:sapphire laser tuned to 925 nm, and the CMTPX-labeled T cells were imaged with the InSight® DS+ tuned to 1080 nm. The experiment follows a single T cell interacting with a dendritic cell for approximately 40 minutes.
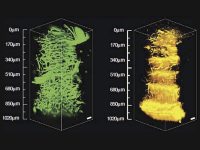
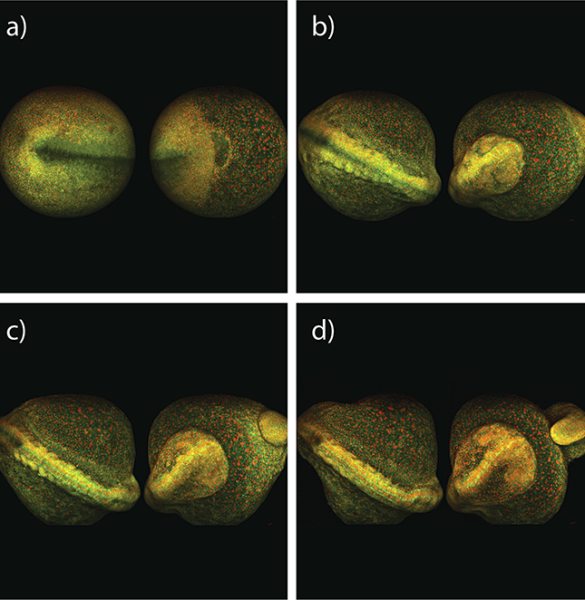
Another experiment tracks the early development of a whole zebra fish embryo using multiphoton imaging. Shown below in Figure 4, Dr. Nadine Peyrieas of the French National Center for Scientific Research in Gif-sur-Yvette, France, tracks the embryo over eight hours using the InSight DS+ Dual. Simultaneous multiphoton imaging performed at 980 nm and 1041 nm was possible using the laser's dual beam configuration. The embryo cell membranes were labeled with eGFP, and the cell nuclei with mCherry.
The early development of a whole zebra fish embryo can be continuously imaged using MPEF microscopy at reduced power levels. In this example, Dr. Nadine Peyrieas of the French National Center for Scientific Research in Gif-sur-Yvette, France, tracked the development over eight hours using a microscope equipped with the InSight DS+ Dual (See figure 4). The embryo cell membranes were labeled with eGFP, and the cell nuclei with mCherry. The eGFP and mCherry were imaged simultaneously at 980 nm and 1041 nm, respectively, using the laser's dual beam configuration. The embryo was imaged from both the front and back sides sequentially.
Multiphoton Imaging Techniques